Ever wondered how the shapes of molecules dictate their behavior, influencing everything from the air we breathe to the medicines that heal us? Understanding the three-dimensional arrangements of atoms, a concept known as molecular geometry, is key to unlocking these mysteries. Within this fascinating realm, trigonal pyramidal geometry stands out as a crucial structure.
In the intricate world of chemistry, the way atoms arrange themselves is far from random. These arrangements define how molecules interact, react, and shape the properties of matter. Trigonal pyramidal geometry is a fundamental molecular shape, playing a significant role in the behavior of compounds like ammonia (NH). This particular geometry comes into play when a central atom bonds with three other atoms and also has one lone pair of electrons.
This article dives into the intricacies of trigonal pyramidal geometry, exploring its features, applications, and significance. By the end, you'll gain a comprehensive understanding of this remarkable molecular structure and its implications in our world.
- Frankie Beverlys Health Battle A Story Of Resilience Hope
- Dog Sideeye Memes Why Theyre So Popular How To Make Yours
In the realm of chemistry, molecular geometry moves beyond abstract theory; it forms the basis of how molecules interact and behave. Trigonal pyramidal geometry is one of the most frequently observed molecular shapes, found in familiar compounds like ammonia (NH). It arises when a central atom is bonded to three other atoms and possesses one lone pair of electrons.
This article will delve into the complexities of trigonal pyramidal geometry, exploring its characteristics, applications, and significance in the chemical world. By the end, you will have a comprehensive grasp of this fascinating molecular structure and its implications in the real world.
| Overview | Details |
|---|---|
| Name | Trigonal Pyramidal Geometry |
| Definition | A molecular shape where a central atom bonds to three other atoms and has one lone pair of electrons. |
| Key Feature | A triangular base formed by three bonded atoms and a lone pair at the vertex, resembling a pyramid. |
| VSEPR Theory | Electron pairs repel each other, dictating the shape to minimize repulsion. Lone pairs occupy more space. |
| Bond Angle | Typically around 107 (less than the ideal tetrahedral angle of 109.5 due to lone pair repulsion). |
| Examples | Ammonia (NH), Phosphine (PH), Chlorate Ion (ClO) |
| Properties | Polarity, increased reactivity (due to the lone pair), and solubility in polar solvents. |
| Applications | Ammonia production, drug design, and catalysis. |
| Biological Significance | Found in enzyme active sites, contributing to protein structure and function. |
| Comparison | Different from tetrahedral and trigonal planar geometries in terms of bond angles and electron arrangement. |
| Impact | Essential for understanding molecular behavior, predicting interactions, and developing new technologies. |
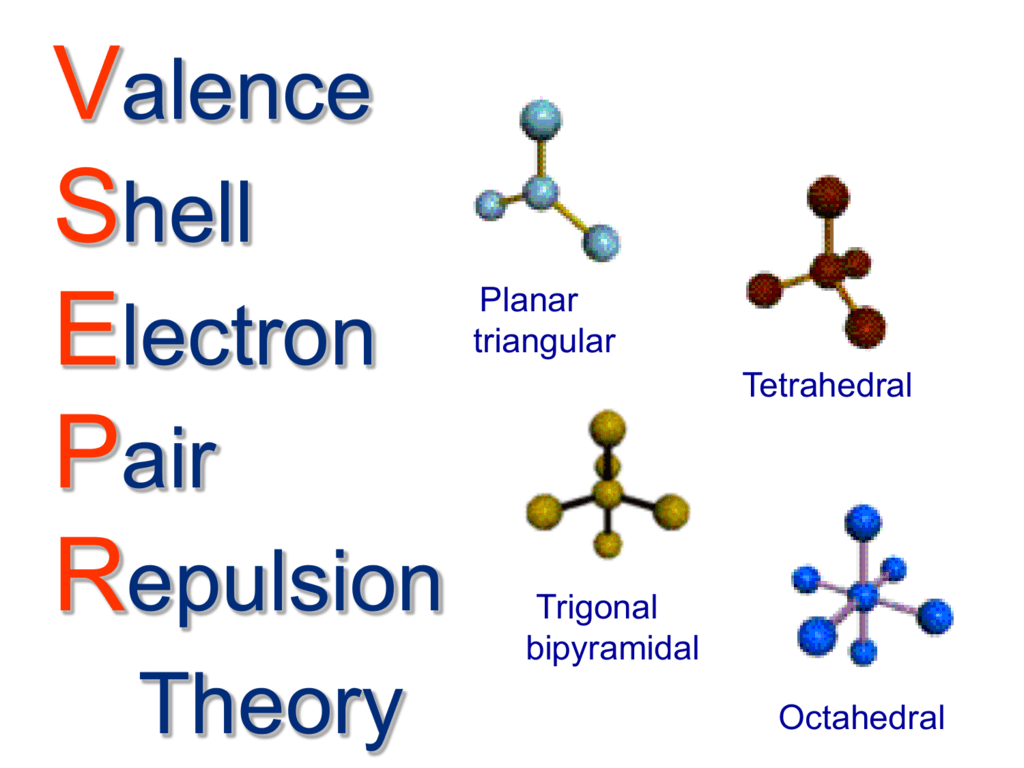
Trigonal pyramidal geometry refers to the three-dimensional arrangement of atoms in a molecule. Here, a central atom is bonded to three other atoms and also holds one lone pair of electrons. This geometry is best understood through the Valence Shell Electron Pair Repulsion (VSEPR) theory, which predicts molecular shapes based on the repulsion between electron pairs.
- Cherie Deville The Step Mom Icons Rise Career Latest News
- Discover The Best Japanese Girl Boxes A Deep Dive
The key features of trigonal pyramidal geometry include a central atom bonded to three surrounding atoms, one lone pair of electrons on the central atom, a triangular base created by the three bonded atoms, and a vertex occupied by the lone pair of electrons. These characteristics result in a molecular shape that resembles a pyramid with a triangular base, hence the name "trigonal pyramidal."
Valence Shell Electron Pair Repulsion (VSEPR) theory is the cornerstone for understanding trigonal pyramidal geometry. According to VSEPR, electron pairs around a central atom repel each other and arrange themselves to minimize this repulsion. In the case of trigonal pyramidal geometry, the lone pair of electrons takes up more space than bonding pairs. This causes the bonded atoms to be pushed closer together.
Lone pairs of electrons exert a significant influence on the molecular shape. They occupy more space than bonding pairs due to their greater electron density, causing the bond angles to deviate from the ideal tetrahedral angle of 109.5. In trigonal pyramidal molecules, the bond angle is typically around 107, a deviation that has major implications for molecular behavior and reactivity.
Trigonal pyramidal geometry is observed in several well-known molecules. Here are some common examples:
Ammonia (NH) is one of the most familiar examples of a trigonal pyramidal molecule. It is composed of a nitrogen atom bonded to three hydrogen atoms and has one lone pair of electrons. The lone pair causes the hydrogen atoms to be pushed closer together, resulting in a bond angle of approximately 107.
Phosphine (PH) is another example of a trigonal pyramidal molecule. It has a similar structure to ammonia, with a phosphorus atom bonded to three hydrogen atoms and one lone pair of electrons. However, the bond angle in phosphine is slightly smaller than in ammonia due to the larger size of the phosphorus atom.
The chlorate ion (ClO) is an example of a trigonal pyramidal molecule, where a central chlorine atom is bonded to three oxygen atoms and has one lone pair of electrons. The lone pair causes the oxygen atoms to be pushed closer together, leading to a bond angle of approximately 101.
The bond angle in trigonal pyramidal molecules is impacted by the repulsion between electron pairs. In an ideal tetrahedral arrangement, the bond angle is 109.5. However, the presence of a lone pair of electrons in trigonal pyramidal molecules causes the bond angle to decrease due to the increased repulsion.
Several factors affect the bond angle, including the size of the central atom, the electronegativity of the surrounding atoms, and the number and type of electron pairs (lone pairs vs. bonding pairs). For instance, ammonia (NH) has a bond angle of roughly 107, while phosphine (PH) has a smaller bond angle because of the larger size of the phosphorus atom.
Trigonal pyramidal molecules show unique properties directly related to their geometry. Most trigonal pyramidal molecules are polar because of the presence of a lone pair of electrons, which creates an uneven distribution of charge. Also, the lone pair of electrons can participate in chemical reactions, making trigonal pyramidal molecules more reactive than molecules with other geometries. Due to their polarity, trigonal pyramidal molecules are often soluble in polar solvents like water.
Trigonal pyramidal geometry is often compared to other molecular geometries like tetrahedral and trigonal planar. Tetrahedral geometry occurs when a central atom bonds to four surrounding atoms and has no lone pairs of electrons. The bond angle in tetrahedral molecules is 109.5, which is larger than the bond angle in trigonal pyramidal molecules because of the absence of lone pairs.
Trigonal planar geometry occurs when a central atom bonds to three surrounding atoms and does not have any lone pairs of electrons. The bond angle in trigonal planar molecules is 120, significantly larger than the bond angle in trigonal pyramidal molecules. Understanding the differences between these geometries is essential for predicting molecular behavior and reactivity.
Trigonal pyramidal geometry has numerous applications in chemistry, biology, and industry. Ammonia, with its trigonal pyramidal structure, is a key component in fertilizers and industrial chemicals, and its geometry is crucial for its reactivity. Many pharmaceuticals contain trigonal pyramidal molecules designed to interact with specific biological targets. In addition, trigonal pyramidal molecules are used as catalysts in chemical reactions because of their reactivity and polarity.
Trigonal pyramidal geometry is essential in chemistry and biology. It explains molecular behavior in different environments and predicts their interactions in chemistry. In biology, trigonal pyramidal molecules are involved in many essential processes like enzyme activity and protein folding.
Trigonal pyramidal molecules are frequently found in biological systems. These molecules participate in essential processes, including enzyme catalysis, where enzymes often contain trigonal pyramidal active sites that bind to specific substrates. In protein structure, the geometry of certain amino acids and nucleotides contributes to the structure and function of proteins and DNA. Understanding the role of trigonal pyramidal geometry in biology is essential for advancing medical research and drug development.
As you've seen, trigonal pyramidal geometry has various applications in chemistry, biology, and industry. Understanding this geometry is essential for grasping the complexities of the chemical world, whether you're a student, researcher, or simply curious about molecular structures.
- Concert Outfit Ideas Style Comfort For Your Next Show
- Ppt Night Ideas To Transform Your Presentations